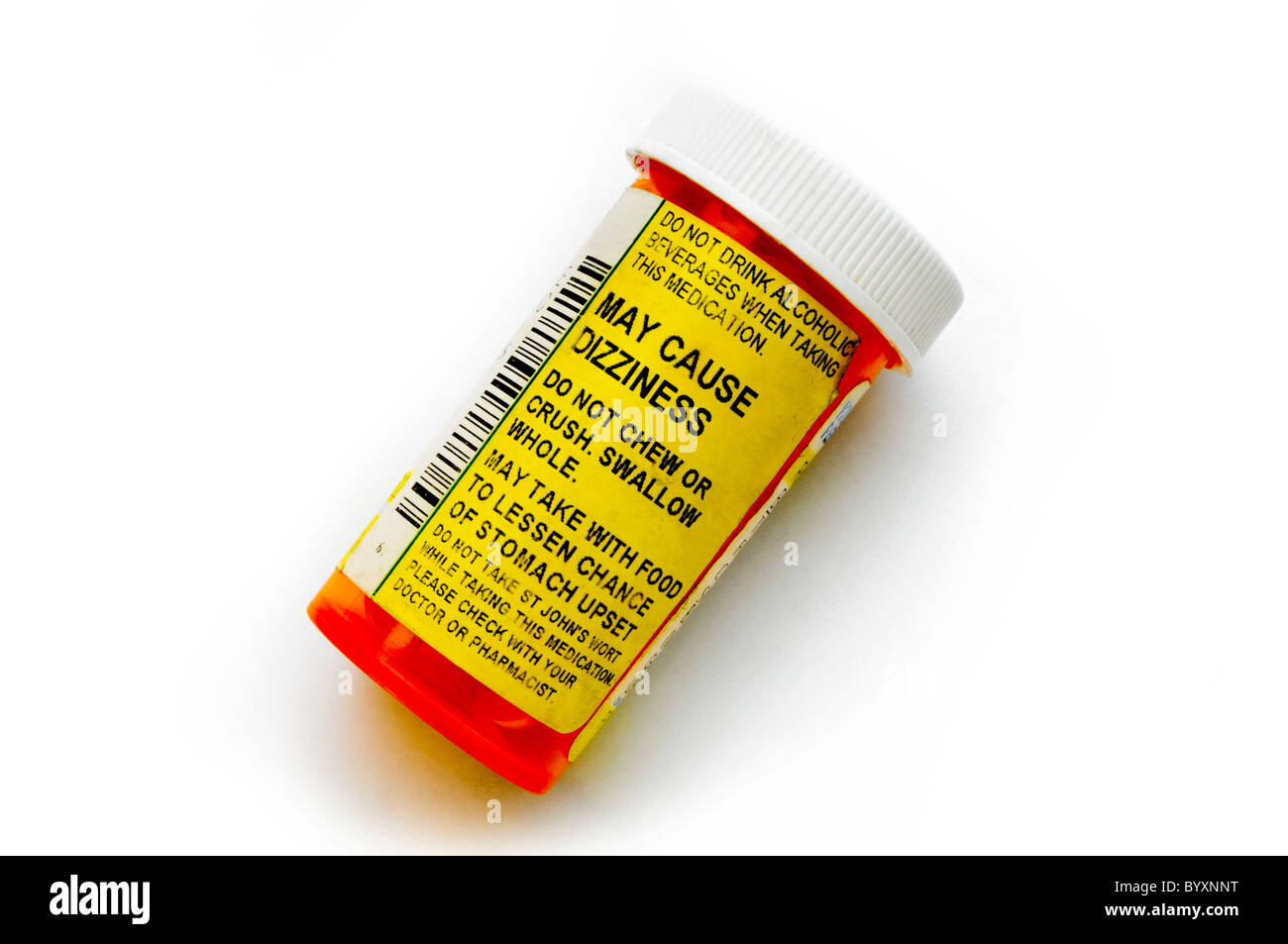
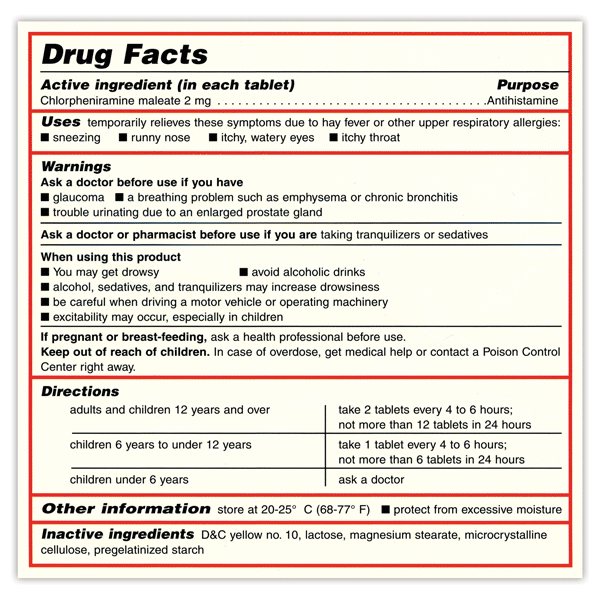
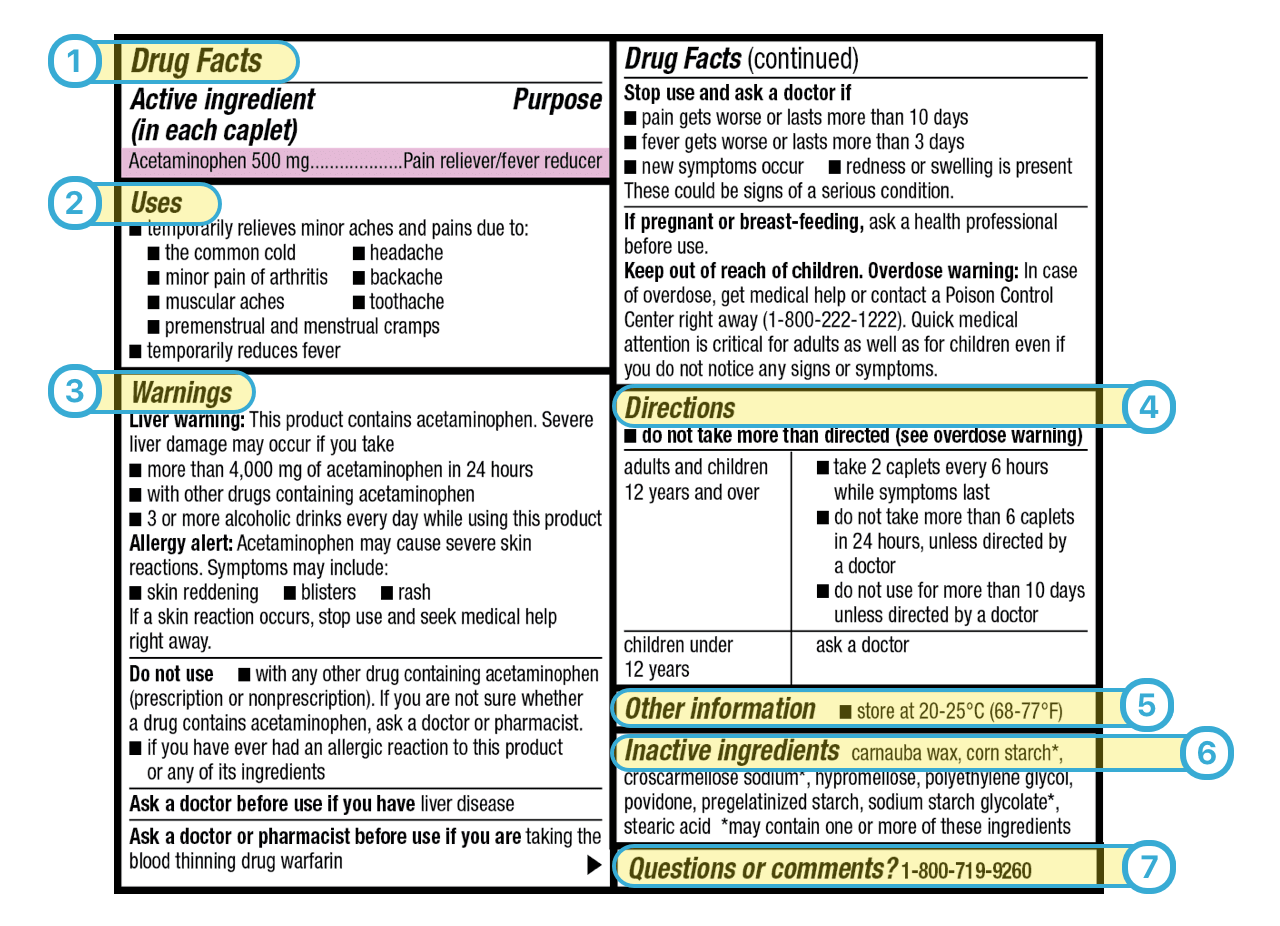
38 warning labels on drugs
FDA Drug Safety Communication: FDA strengthens warning that ... The U.S. Food and Drug Administration (FDA) is strengthening an existing label warning that non-aspirin nonsteroidal anti-inflammatory drugs (NSAIDs) increase the chance of a heart attack or stroke. FDA Drug Safety Communication: FDA cautions about using ... The testosterone product labels have been updated. The revised labels clarify the approved uses of these medications and include information about a possible increased risk of heart attacks and ...
Alcohol Warning Labels Need an Update, Researchers Say Sep 01, 2022 · Sept. 1, 2022 – Warning labels on alcoholic drinks need to be updated to spell out details of potential harm in order to make them more effective, two U.S. researchers say.

Warning labels on drugs
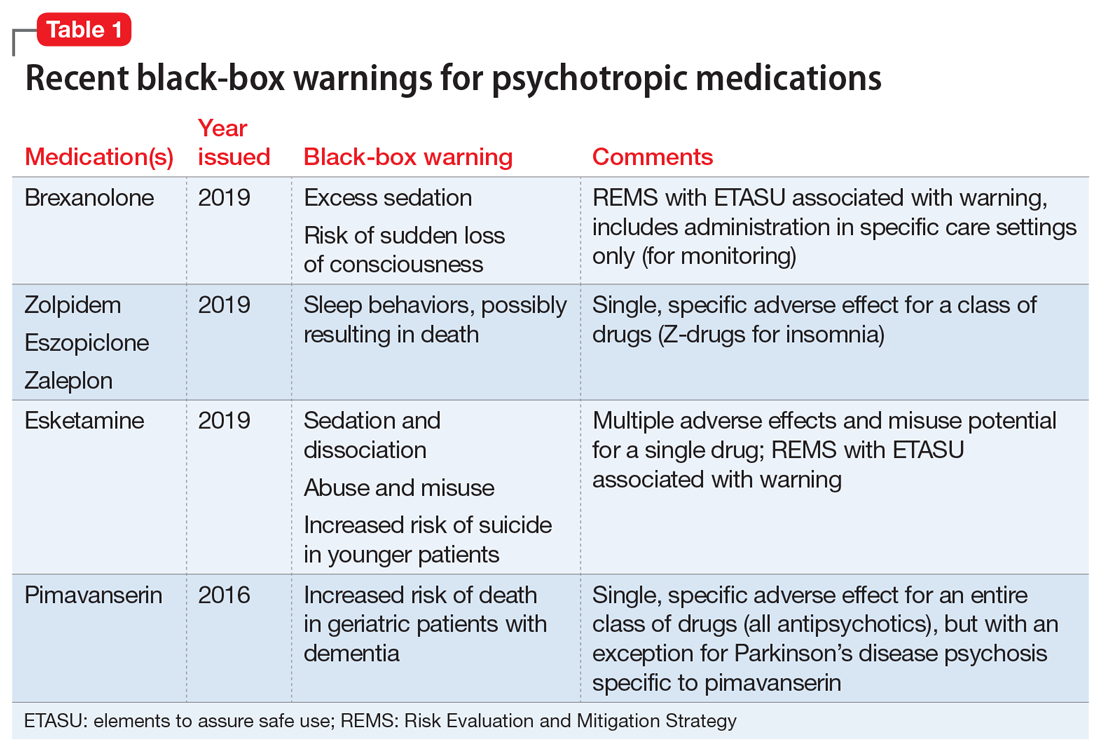
Black Box Drugs | FDA Warning Information - ConsumerSafety.org Dec 08, 2017 · A boxed warning is the FDA's harshest warning, and the last stop before a recall notice. A study from 1998 broke down the criteria for placing a boxed warning on a medication. The researchers noted that the FDA might have one or more of the following reasons to add a boxed warning: FDA revises labels of SGLT2 inhibitors for diabetes to ... Mar 15, 2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ... FDA updates warnings for oral and injectable fluoroquinolone [07-26-2016] The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection).
Warning labels on drugs. Boxed warning - Wikipedia In the United States, a boxed warning (sometimes "black box warning", colloquially) is a type of warning that appears on the package insert for certain prescription drugs, so called because the U.S. Food and Drug Administration specifies that it is formatted with a 'box' or border around the text. FDA updates warnings for oral and injectable fluoroquinolone [07-26-2016] The U.S. Food and Drug Administration (FDA) approved changes to the labels of fluoroquinolone antibacterial drugs for systemic use (i.e., taken by mouth or by injection). FDA revises labels of SGLT2 inhibitors for diabetes to ... Mar 15, 2022 · A U.S. Food and Drug Administration (FDA) safety review has resulted in adding warnings to the labels of a specific class of type 2 diabetes medicines called sodium-glucose cotransporter-2 (SGLT2 ... Black Box Drugs | FDA Warning Information - ConsumerSafety.org Dec 08, 2017 · A boxed warning is the FDA's harshest warning, and the last stop before a recall notice. A study from 1998 broke down the criteria for placing a boxed warning on a medication. The researchers noted that the FDA might have one or more of the following reasons to add a boxed warning:








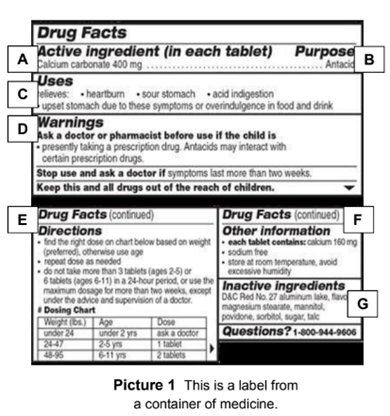
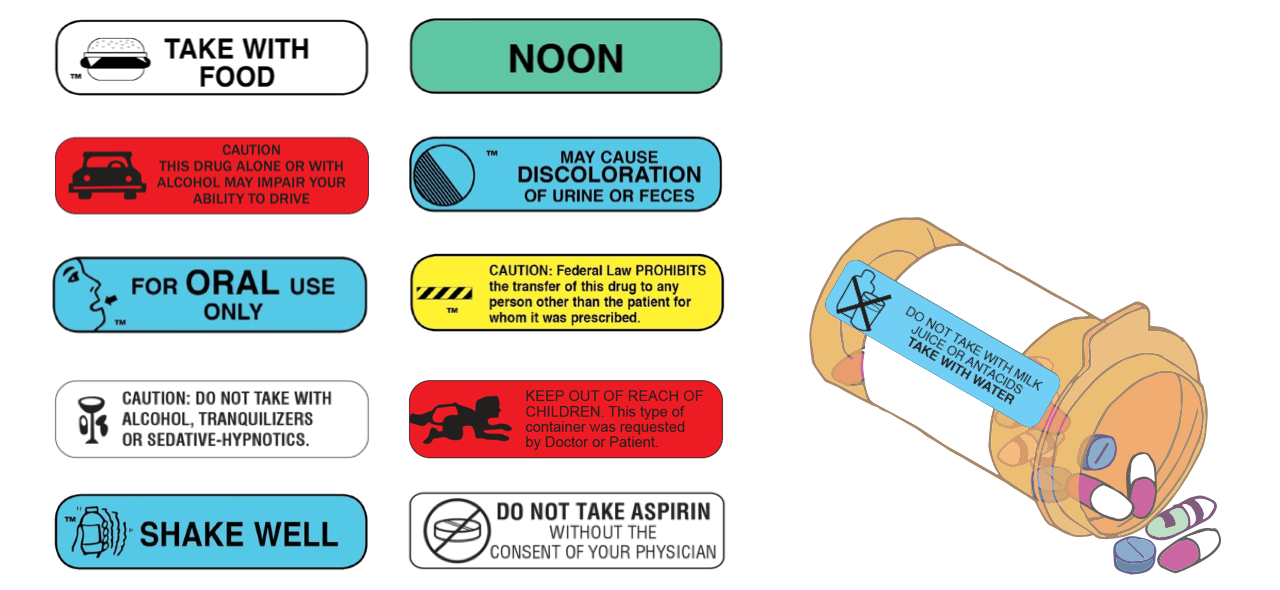

![PDF] Improving prescription drug warnings to promote patient ...](https://d3i71xaburhd42.cloudfront.net/e087ba4ebaa8ed043bf22bd2ab63292d6a05b9d4/3-Figure2-1.png)
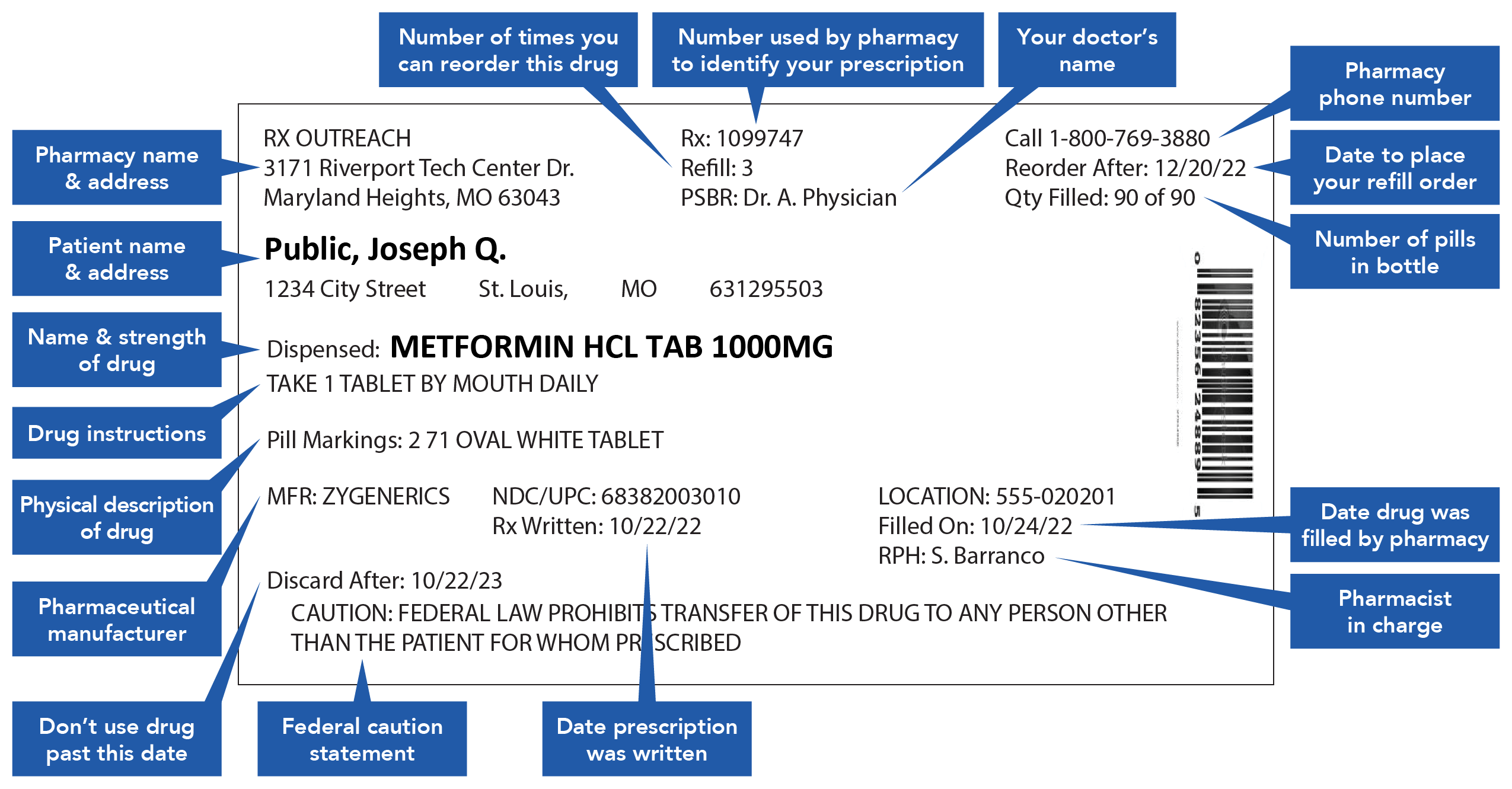

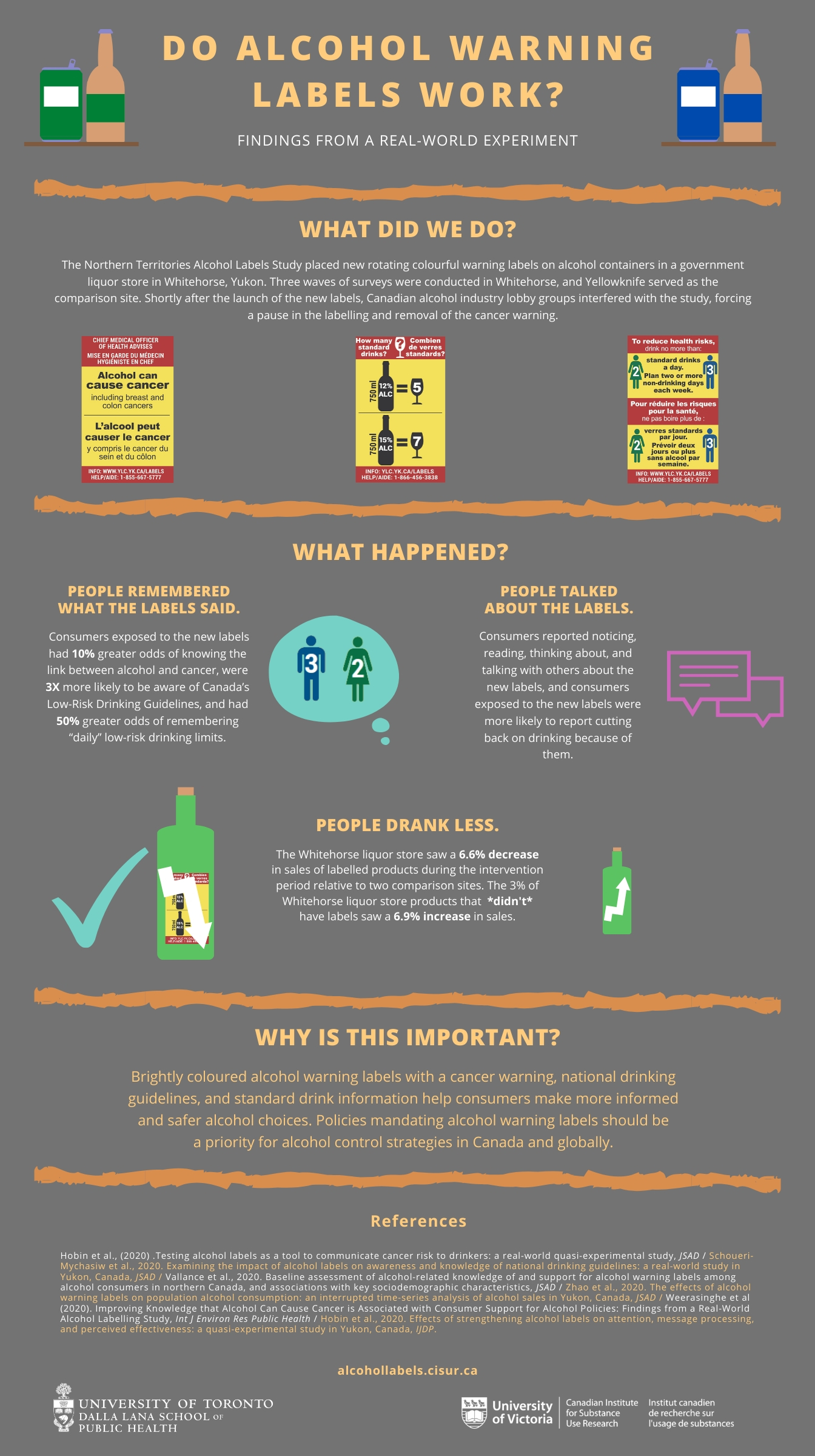





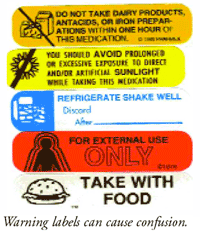








Post a Comment for "38 warning labels on drugs"