39 phase labels in chemical equations
Answered: Complete and balance the following… | bartleby Transcribed Image Text: Complete and balance the following molecular equations (indicate the relevant phase labels) LICI (s) + HSO4 (aq) →→ NH₂ (aq) + OH(aq) → AgNOs (aq) + + (aq) →> NaHCOs (aq) + H* (aq) → 0 0 0. Expert Solution. ... Using chemical equations, show how the triprotic acid H₂PO4 ionizes in water. Phases are optional Chemical Phase Equilibria and Phase Diagrams | SpringerLink The representation of chemical phase equilibria in ternary systems is significantly more challenging than binary systems. ... Find the equation describing the activity coefficient of A in the liquid along the liquidus line as a function of composition and temperature. ... Label all of the two-phase coexistence regions and three-phase ...
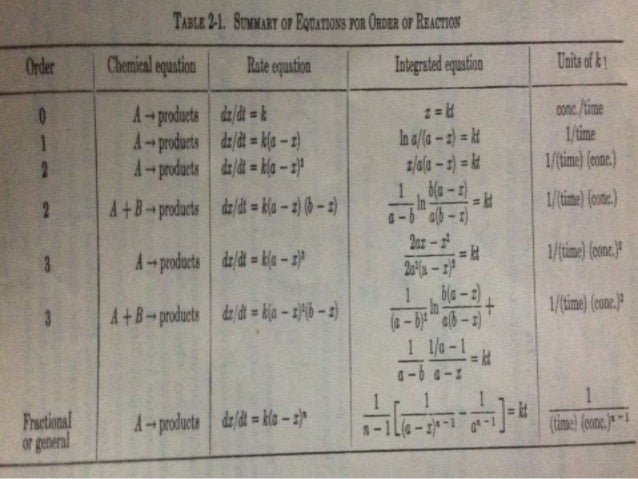
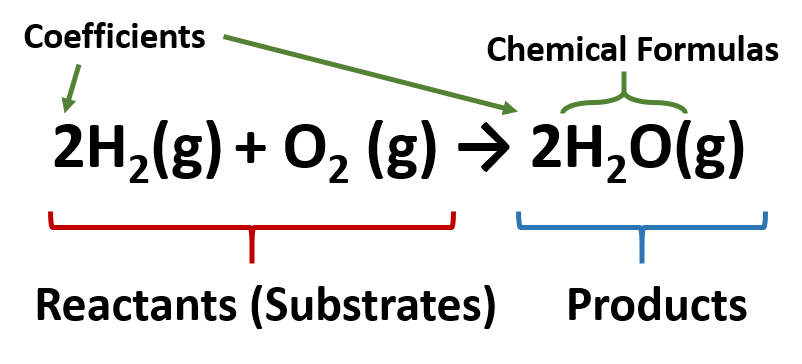
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways
Phase labels in chemical equations
Gibbs' Phase Rule: Where it all Begins - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases. Answered: Complete and balance the three… | bartleby Solution for Complete and balance the three equations according to acid and base behavior in water. Phase labels are optional. HNO, (aq) + H,O(1) H,0 Ba(ОН),… The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O(ℓ)
Phase labels in chemical equations. End-of-Chapter Material | Introductory Chemistry - Lumen Learning Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why. H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Definition of dissolve and the state label (aq) in chemical equations C O X 2 ( a q) would imply dissolved carbon dioxide in water (i.e.,to our eyes it is a single phase, just like an unopened bottle of coke). B r X 2 ( a q) would imply homogeneous solution of liquid bromine in water. N a C l ( a q): Salt dissolved in water. A colloid is a heterogeneous phase. 3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g)
End-of-Chapter Material - GitHub Pages Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Solved 1. Write balanced chemical equations using proper | Chegg.com Chemistry questions and answers. 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust. b) Solid calcium carbonate is added to a solution of hydrochloric acid, yielding an aqueous solution ... The Chemical Equation - GitHub Pages In chemical equations, the number of atoms of each element in the reactants must be the same as the number of atoms of each element in the products. ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as ... What are Chemical Equations? Detailed Explanation, Examples - BYJUS An example of an ionic chemical equation is provided below. Chemical Equation: CaCl 2 + 2AgNO 3 → Ca (NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3- → Ca 2+ + 2NO 3- + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ ( calcium ion ...
5.2 Chemical Equations - Lumen Learning It is not uncommon to include a phase label with each formula—(s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for a substance dissolved in water, also known as an aqueous solution. If we included phase labels for the reactants and products, under normal environmental conditions, the reaction would be as follows: H 2 (g) + O 2 (g) → H 2 O(ℓ) End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Phase Definition and Examples - ThoughtCo A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter. The states of matter (e.g., liquid, solid, gas) are phases, but matter can exist in different phases yet remain in the same state of matter. For example, liquid mixtures can exist in multiple phases, such ... 5.7 End-of-Chapter Material - Introductory Chemistry - NSCC Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
chemical equation | Yeah Chemistry In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution For example; Burning of sodium in chlorine produces sodium chloride.
Solved 2. Chemical equations can also be used to represent | Chegg.com Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. 3. Explain why 4Na(s) + 2C12(9) - 4NaCl(s) should not be considered a proper chemical equation. 4. Explain why H2(g) + 1/2O2(g) → H20(1) should not be considered a proper chemical equation. 5.
State symbols and phase changes | StudyPug A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l) Gas, given the symbol (g) Aqueous, meaning dissolved in water, and given the symbol (aq)
Conventions for Writing Chemical Equations - Arrows, Phases ... presents: Conventions for Writing Chemical Equations including Arrows, Phases, Coefficients, Reactants, Products and moreTired...
DOC Chapter 2 Identify the reactants and products in a chemical equation. Write chemical equations using appropriate phase labels, symbols of reaction conditions, and the presence of a catalyst. 2.10 Balancing Chemical Equations. Learning Objectives. Determine if a chemical reaction is balanced. Master techniques for balancing chemical equations. (Example 2.12)
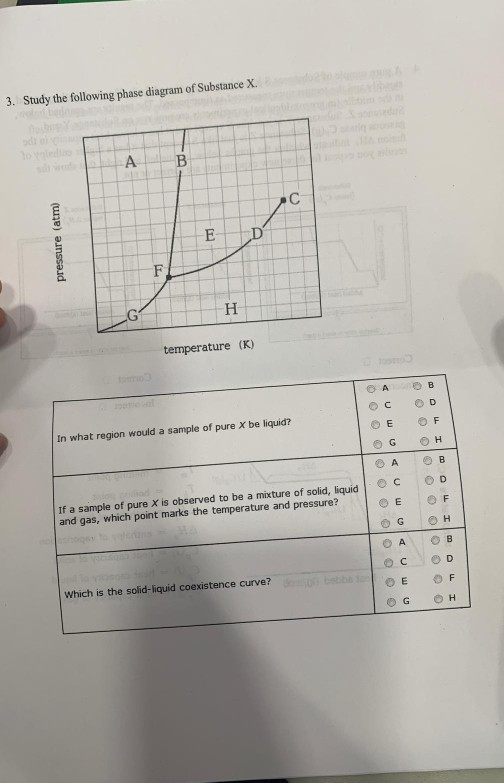
Phase Diagram | Explanation, Definition, Summary & Facts The first curve line (A, T) separated the solid and vapour phase, second curve line (T, B) separates the solid and liquid phase whereas third curve (T, C) separates the liquid and vapour phases of a substance (Fig. 1).
EXAM #2: Chapter 4 Flashcards | Quizlet Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. ... 3. Explain why 4Na (s)+2ClX2 (g) 4NaCl (s) should not be considered a proper chemical equation. ... 4. Explain why HX2 (g)+12OX2 (g) HX2O (ℓ)
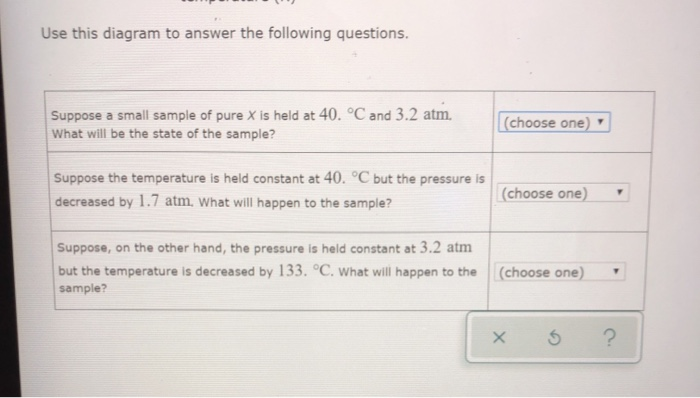
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water, we can determine that the state of water at each temperature and pressure given are as follows: (a) solid; (b) liquid; (c) liquid; (d) gas; (e) solid; (f) gas. Check Your Learning What phase changes can water undergo as the temperature changes if the pressure is held at 0.3 kPa? If the pressure is held at 50 kPa?
6.3 Acid-Base Reactions - CHEM 1114 - Introduction to ... - BCcampus The process represented by this equation confirms that hydrogen chloride is an acid. When dissolved in water, H 3 O + ions are produced by a chemical reaction in which H + ions are transferred from HCl molecules to H 2 O molecules ().. Figure 1. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and ...
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation. Does the chemical reaction represented by 3Zn (s) + 2Al (NO 3) 3 (aq) → 3Zn (NO 3) 2 (aq) + 2Al (s) proceed as written?
A thermochemical equation is a balanced chemical reaction equation ... An equation which shows both mass and heat relationships between products and reactants is called a thermochemical equation. The following four reactions are examples of thermochemical equations. The first two are exothermic and the last two are endothermic reactions. 2 H 2 (g) + O 2 (g) ----> 2 H 2 O(l) DH = -571.6 kJ
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O(ℓ)
Answered: Complete and balance the three… | bartleby Solution for Complete and balance the three equations according to acid and base behavior in water. Phase labels are optional. HNO, (aq) + H,O(1) H,0 Ba(ОН),…
Gibbs' Phase Rule: Where it all Begins - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases.










Post a Comment for "39 phase labels in chemical equations"